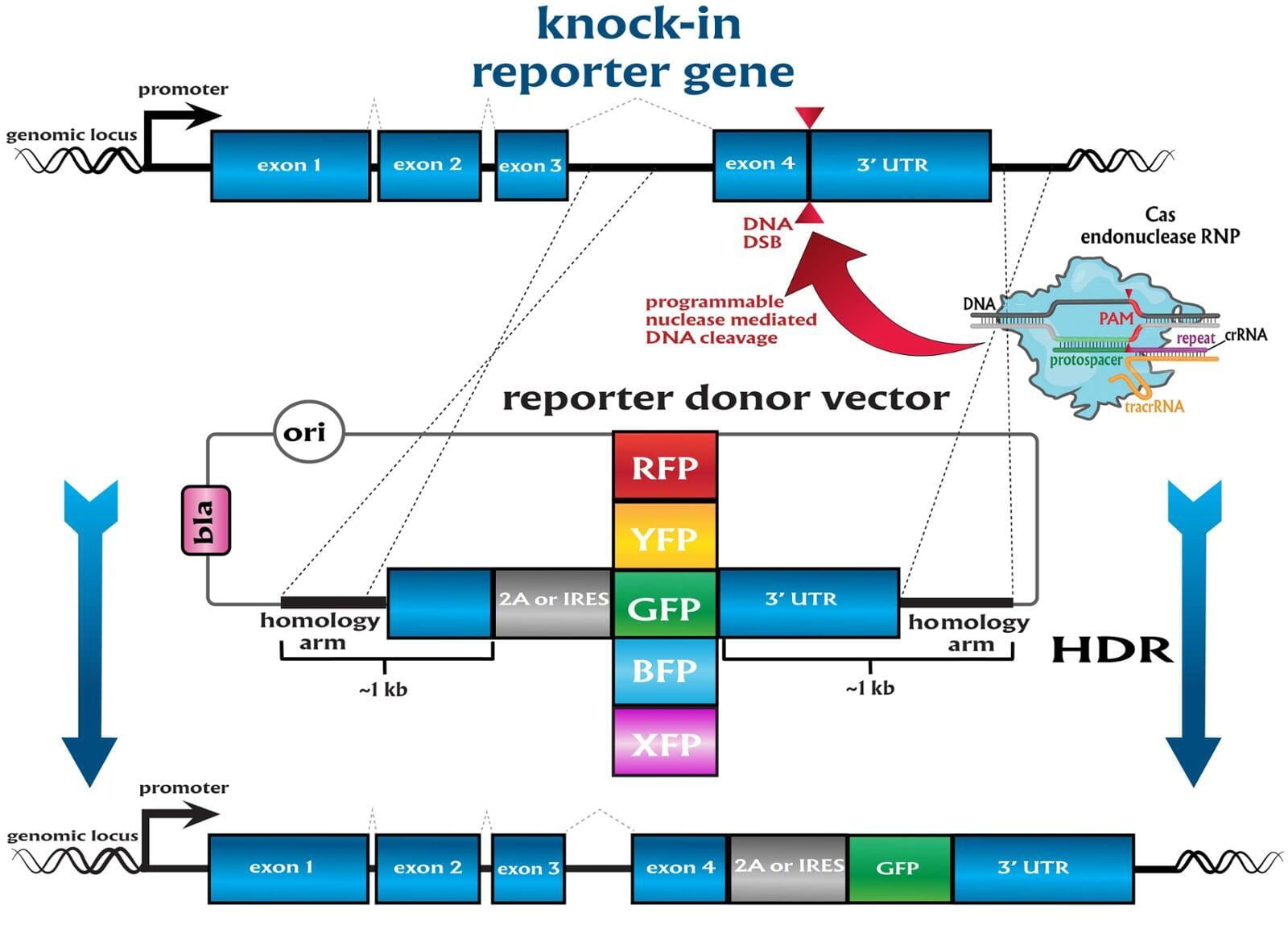
knock-in (KI)
Service
Catalog Nr
Service Description
Timeline
Deliverables
Pricing
- Rosa26 safe harbor knockin
- C57BL/6 mouse KI via CRISPR microinjection
- guaranteed founders
At least 2 KI founders (or germline transmitted F1s) with the confirmed mutation
- Rosa26 safe harbor knockin
- C57BL/6 mouse KI via CRISPR microinjection
- non-guaranteed service (per injection session)
Services included are insertion of point mutations, altering amino acid codons, insertion of tags and barcodes, reporter gene knockins (GFP, mVenus, mCherry) and whole gene replacement such as humanization. Microinjection is performed as a per session service with a total of 500 zygotes injected.
At least 500 embryos will be injected and implanted (2-3 microinjection sessions), which usually results in approximately 100 pups. Ear biopsies from all pups will be given to the investigator for genotyping. There is no guarantee that resulting pups will be gene edited.
- Rosa26 safe harbor knockin
- custom mouse strain - CRISPR-mediated knockin
- guaranteed founders
At least 2 KI founders (or germline transmitted F1s) with the confirmed mutation
At least 2 KI founders (or germline transmitted F1s) with the confirmed mutation
- Custom knockin any locus
- C57BL/6 mouse KI via CRISPR microinjection
- non-guaranteed service (per injection session)
At least 500 embryos will be injected and implanted (2-3 microinjection sessions), which usually results in approximately 100 pups. Ear biopsies from all pups will be given to the investigator for genotyping. There is no guarantee that resulting pups will be gene edited.
- Custom knockin any locus
- custom mouse strain - CRISPR-mediated knockin
- guaranteed founders
At least 2 KI founders (or germline transmitted F1s) with the confirmed mutation
Service
Catalog Nr
Service Description
Timeline
Deliverables
Pricing
- Gene editing activity testing
- Format - cell-based transfection
- Assay - T7 endonuclease I/Cel-II/Surveyor
Activity of up to 6 guide RNAs
- Gene editing activity testing
- Format - cell-based transfection
- Assay - genetic reporter & flow cytometry
Activity of up to 6 guide RNAs
genotyping protocol
genotyping protocol





